- Visibility 127 Views
- Downloads 21 Downloads
- DOI 10.18231/j.ijmpo.2021.014
-
CrossMark
- Citation
Evaluation of peripheral lymphadenopathy by fine needle aspiration cytology: Our experience at a tertiary care center
- Author Details:
-
Syed Fiza Mustaqueem *
-
Syed Belal Hassan
-
Shikha Agarwal
Introduction
Peripheral lymph nodes, located deep in the subcutaneous tissue are an important part of immune system as they clean antigens from the extracellular fluid.[1] There are diverse etiologies of peripheral lymphadenopathy, ranging from benign reactive conditions (eg viral or bacterial infections including tuberculosis) to metastatic carcinomas and lymphomas.[2] As the enlarged lymph nodes are easily accessible for fine needle aspiration cytology (FNAC), it permits rapid diagnosis with minimum intervention.[3] Thus it allows a provisional diagnosis to be made at the patient’s initial presentation and helps to guide appropriate specialist referral and further investigations.
Aspiration of lymph node for diagnostic purpose was first reported in 1904 by Grieg & Gray in the diagnosis of trypanosomiasis. In 1921, Guthrie attempted to correlate lymph node aspiration cytology with various disease processes.[4], [5], [6]
The dilemma to approach a patient with peripheral lymphadenopathy, its evaluation and management, considering various differential diagnosis, prompted us to take up this study. We conducted this study to take evaluate the role of FNAC in the diagnosis of peripheral lymphadenopathy and to study the pattern and spectrum of diseases associated with them in different age groups and sex in this geographical area.
Materials and Methods
All FNA performed on patients with palpable lymph node enlargement in the Department of Pathology, Hind Institute of Medical Sciences, Barabanki between January 2018 to December 2019 were included in the study. A total of 331 cases were included in the study; out of which 176 were retrospective and 155 prospective. Relevant data including age, sex, clinical presentation and other relevant investigations were retrieved from records. The cytology smears of retrospective cases were retrieved from records of pathology department. In the prospective cases, aspirates were performed by cytology staff using 22-24 gauge needle by non-aspiration/aspiration technique. Routinely 2 aspirates were performed and direct smears were prepared. Slides were immediately immersed in 95% methanol for wet fixation and the remaining slides were air dried for dry fixation and stained with Haematoxylin and Eosin and Papanicolaou stains. Special stains like Ziehl Neelsen (ZN) stain for acid fast bacilli were used wherever required. Stained smears were examined under the microscope and results were analysed to study the spectrum of pathologies reported on FNAC.
At the end of study period, the data was analyzed using Advanced Excel software. Fischer’s exact test was used to check the association of age and sex with the cytodiagnosis. P-value ≤ 0.05 was considered as significant.
Results
This study analysed 331 cases of lymphadenopathy during the period January 2018 to December 2019. The age range of the patients was from 6 months to 75 years in which 58.3% were males and 41.7% were females. These cases were divided into 4 groups according to their age: group I (≤15 years), group II (16-35 years), group III (36-50 years) and group IV (>50 years). The maximum number of cases were in the age group ≤15 years.
It was observed that cervical region was the most common site of lymphadenopathy (67.07%), followed by axillary (11.18%), inguinal (9.97%) and supraclavicular (8.76%). 10 cases (3.02%) showed generalised lymphadenopathy.([Table 1])
|
Lymph node group |
No. of Cases |
Percentage |
|
Cervical |
222 |
67.07 |
|
Axillary |
37 |
11.18 |
|
Supraclavicular |
29 |
8.76 |
|
Inguinal |
33 |
9.97 |
|
More than 1 group involved |
10 |
3.02 |
|
Total |
331 |
100 |
The cytomorphological patterns of lymph node lesions showed non-neoplastic lesions (83.69%) to be more common as compared to neoplastic lesions (16.31%). Out of 331 cases, maximum cases (38.67%) were of granulomatous lymphadenitis; followed by non-specific reactive lymphadenitis (36.25%) and suppurative lymphadenitis (8.76%). ([Table 2])
Out of 128 cases of granulomatous lymphadenitis, ZN stain was used in every case and showed the presence of acid fast bacilli in 78/128 (60.94%) cases, whereas 50/128 (39.06%) cases did not reveal acid fast bacilli.
|
Cytodiagnosis |
No. of Cases |
Percentage |
|
Non-neoplastic lesions |
||
|
Non-specific reactive Lymphadenitis |
120 |
36.25 |
|
Granulomatous Lymphadenitis |
128 |
38.67 |
|
Suppurative Lymphadenitis |
29 |
8.76 |
|
Neoplastic Lesions |
||
|
Metastatic Carcinoma |
43 |
12.99 |
|
Non Hodgkin’s Lymphoma |
8 |
2.41 |
|
Hodgkin’s Lymphoma |
3 |
0.90 |
Amongst the neoplastic lesions, metastatic carcinoma was the most commonly seen lesion (12.99%); as compared to 8 cases (2.41%) of NHL and only 3 cases (0.90%) of HL. The metastatic lesions showed squamous cell carcinoma (62.79%) more frequent than poorly differentiated carcinoma (25.58%) and adenocarcinoma (11.63%). ([Table 3] )
|
Metastatic Lesion |
No. of cases |
Percentage |
|
Squamous cell carcinoma |
27 |
62.79 |
|
Adenocarcinoma |
5 |
11.63 |
|
Poorly differentiated carcinoma |
11 |
25.58 |
|
Diagnosis on FNAC |
≤ 15 years |
15 to 35 years |
36 to 50 years |
> 50 years |
p-value |
|
Non-specific reactive Lymphadenitis |
64 |
25 |
20 |
11 |
<0.05 |
|
Granulomatous Lymphadenitis |
42 |
55 |
23 |
8 |
|
|
Suppurative Lymphadenitis |
11 |
10 |
4 |
4 |
|
|
Lymphoproliferative disorder |
- |
2 |
3 |
6 |
|
|
Metastatic Carcinoma |
- |
1 |
15 |
27 |
Agewise distribution of cytomorphological patterns of diagnosed cases showed that non-specific reactive (53.33%) and suppurative lymphadenitis (37.93%) were more commonly seen in ≤15 years. Granulomatous lymphadenitis (42.96%) was more common in 15-35 years age group. Amongst the neoplastic lesions, metastatic carcinoma (62.79%) and NHL (75%) were more common in >50 years. 2/3 cases (66.67%) of HL were seen in 15-35 years age group. ([Table 4])
|
Diagnosis on FNAC |
Male |
Female |
p-value |
|
Non-specific reactive Lymphadenitis |
83 |
37 |
<0.05 |
|
Granulomatous Lymphadenitis |
55 |
73 |
|
|
Suppurative Lymphadenitis |
18 |
11 |
|
|
Lymphoproliferative disorder |
8 |
3 |
|
|
Metastatic Carcinoma |
29 |
14 |
Genderwise distribution of cytomorphological patterns of diagnosed cases showed that non-specific reactive 83/120 (69.17%) and suppurative lymphadenitis 18/29 (62.07%) were more common in males as compared to granulomatous lymphadenitis 73/128 (57.03%) which was more common in females. 29/43 cases (67.44%) of metastatic carcinoma were seen in males. 6/8 (75%) cases of NHL and 2/3 cases (66.67%) of HL were seen in males. ([Table 5])
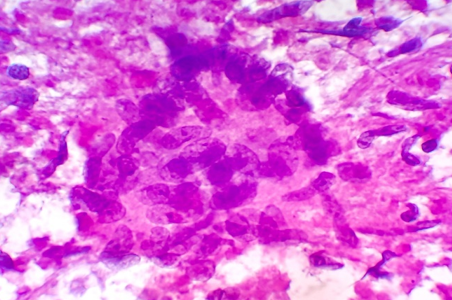
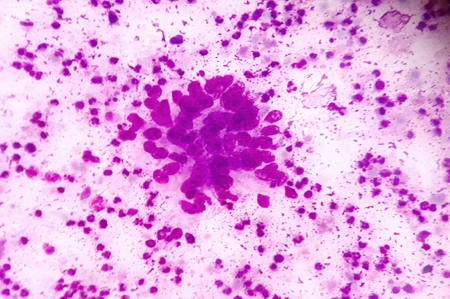
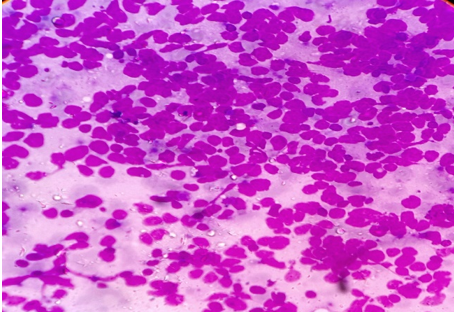
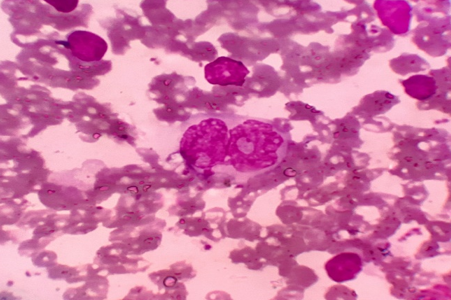
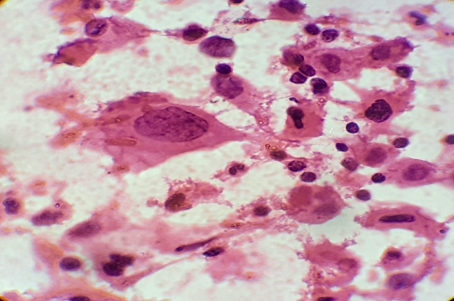
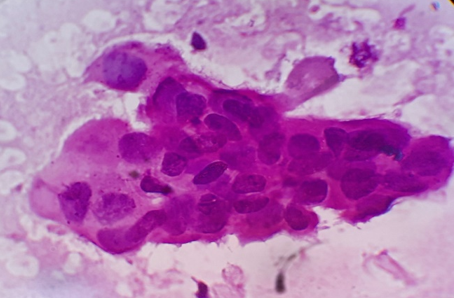
Discussion
FNAC is a valuable diagnostic technique in the evaluation of cases of superficial lymphadenopathy. The categorization of the causes of lymphadenopathy into reactive, inflammatory and lymphoproliferative could be reliably done by FNAC. A high sensitivity (71.4%) and specificity (91.5%) of FNAC was reported in previous studies for the evaluation of lymphadenopathy.[7] The use of this technique has limited the need for excision of enlarged lymph nodes, especially in cases of reactive and tubercular lymphadenitis where conservative management can be offered to patient just on the basis of cytological diagnosis. However potential cytological misdiagnosis may occur, either in lymphomas that present an apparently admixed cell pattern (false negative cases) or in reactive proliferations in which atypical cells are identified (false positive cases).[8] In such cases, the cytological diagnosis should be followed by histological evaluation for accurate diagnosis and subtyping and grading in cases of primary lymphoproliferative disorders.
In our experience of 331 cases, the pattern of non-neoplastic lesions consists of reactive hyperplasia, granulomatous lymphadenitis and suppurative lymphadenitis. Malignant lesion includes metastatic carcinoma, non-Hodgkin’s lymphoma (NHL) and Hodgkin’s lymphoma (HL).
The age group which was studied ranged from 6 months to 75 years with maximum cases in ≤15 years which is comparable with the study of Pandey P et al. However Shilpa G et al, Patel MM et al and Kumar H et al.[9], [10], [11], [12] found maximum number of cases in the age group 21-30. The variation may be due to different population density or geographic variation.
In the present study, the incidence in male (58.3%) was more than that in females with male to female ratio (1.4:1). Similar male preponderance was noted by Patel MM and Kumar N et al.[10], [11]
The most frequent site of FNAC was cervical region (67.07%), followed by axillary region (11.18%). Similar findings were also observed by Wilkinson AR et al, Shah PC et al and Mohanty R et al.[13], [14], [15] The reason behind this may be the easy accessibility of cervical lymph nodes for examination and evaluation.
Cytomorphologic patterns obtained in this study were predominantly non-neoplastic (83.69%) as compared to neoplastic lesions (16.31%). Amongst the non-neoplastic lesions, granulomatous lymphadenitis was the most common pattern (46.21%), followed by non-specific reactive lymphadenitis (43.32%) and suppurative lymphadenitis (10.47%). Similar findings were observed by Shilpa G et al, Shah PC et al and Wahid F et al.[9], [14], [16] This high rate of granulomatous lymphadenitis is because of high incidence of tuberculosis in this geographical area. However Pandey P et al, Mohanty R et al and Shrivastav A et al[12], [15], [17] found reactive lymphadenitis as the most common cytological pattern.
In our study, granulomatous lymphadenitis was most frequently seen in 15-35 years age group; whereas non-specific reactive lymphadenitis was more commonly seen in ≤15 years. Shrivastav A et al[17] have shown similar findings in their study. However study by Thomas JO et al[18] shows tubercular lymphadenitis to be more common in ≤20 years age group. This may be due to difference in population density.
Amongst the neoplastic lesions, the most common lesion was of metastatic carcinoma (43/54); followed by NHL (8/54). Only 3 cases of HL were detected in this study.
Amongst the metastatic lesions, squamous cell carcinoma was the most common variant seen. A similar finding was reported by Shah PC et al, Mamatha K et al and Mohan A et al.[19], [20] This correlates with the higher incidence of upper aerodigestive tract malignancy in India, possibly due to habbit of tobacco chewing in Indian population. However, Ghartinagar et al[21] showed relatively higher incidence of metastatic adenocarcinoma in their study.
Most common age group presenting with metastatic carcinoma was ≥50 years (27/43); followed by 35-50 years. This finding was in concordance with the finding of Ghartinagar et al[21] and Agarwal et al.[22] The possible reason may be because advanced age group is itself a risk factor for carcinoma.
Lymphoproliferative disorders formed a very small proportion (3.31%) of the total pathologies reported, a finding which correlates with other studies.[9], [13], [14] However Dowerah et al[23] reported a higher incidence of 10.6% cases of lymphoma in their series. This variation may be due to difference in demographics, environmental and genetic influence.
We further advised histopathological examination in these cases, but excision biopsy was done in only 5 cases – 4 NHL and 1 HD; which showed concordant results. Rest of the cases were lost in follow up.
Conclusion
To summarize, FNAC is a valuable and reliable screening tool in outpatient department. It helps to categorize the patients into 2 groups; one which can be treated conservatively without requiring histopathological assessment and the other which requires further hisopathological assessment and other ancillary tests for diagnosis. Hence the use of this rapid technique allows a provisional diagnosis to be made at patient’s initial presentation and helps to guide the clinician to manage the patients accordingly. Our statistical data suggests the role of different etiologies in different age groups varying from reactive to metastatic. So it is emphasised that we must keep in mind the higher possibility of neoplastic lesions in ≥50 years age group and should take up ancillary studies on the cytological samples itself for definite diagnosis. This will not only save the time, but is also cost effective; a point to be considered in a developing country like ours.
Conflict of Interest
The authors declare that there are no conflicts of interest in this paper.
Source of Funding
None.
References
- R Ferror. Lymphadenopathy: differential diagnosis and evaluation. Am Fam Physician 1998. [Google Scholar]
- AA Pandit, FP Candes, SR Khubchandani. Fine needle aspiration cytology of lymph nodes. J Postgrad Med 1987. [Google Scholar]
- AR Wilkinson, SA Maimoon, SD Mahore. FNAC in the diagnosis of lymph node malignancies: A simple and sensitive tool. Indian J Med Paediatr Oncol 2012. [Google Scholar] [Crossref]
- N Berliner. Approach to the patient with lymphadenopathy. Best Pract Med 2000. [Google Scholar]
- RK Narang, S Pradhan, RP Singh, S Chaturvedi. Place of fine needle aspiration cytology in the diagnosis of lymphadenopathy. Ind J Tub 1990. [Google Scholar]
- ED Grieg, AC Gray. Lymphatic gland in sleeping sickness. Br Med J 1904. [Google Scholar]
- RO Faro, AZ Mohammed, AT Atanda. Diagnostic utility of fine needle aspiration cytology in the evaluation of peripheral lymphadenopathy. West Afr J Med 2018. [Google Scholar]
- CJ Stewart, JA Duncan, M Farquharson, J Richmond. Fine needle aspiration cytology diagnosis of malignant lymphoma and reactive lymphoid hyperplasia. J Clin Pathol 1998. [Google Scholar] [Crossref]
- G Shilpa, G Nataraju. Pattern of Lymph Node Diseases in a Tertiary Level Referral Center: a cytological study of 943 cases. Int J Biol Med Res 2013. [Google Scholar]
- MM Patel, SL Italiya, RD Patel, RB Dudhat, KR Kaptan, VM Baldwa. Role of Fine Needle Aspiration Cytology to Analyze Various Causes of Lymphadenopathy. Natl J Community Med 2013. [Google Scholar]
- H Kumar, PM Pagaro, AC Buch, SS Chandanwale, CR Gore, VH Satav. Role of fine needle aspiration cytology in assessment of cervical lymphadenopathy. Med J Dr. D.Y. Patil Univ 2013. [Google Scholar] [Crossref]
- P Pandey, A Dixit, NC Mahajan. The diagnostic value of FNAC in assessment of superficial palpable lymph nodes: a study of 395 cases. Al Ameen J Med Sci 2013. [Google Scholar]
- AR Wilkinson, SA Maimoon, SD Mahore. FNAC in the diagnosis of lymph node malignancies: A simple and sensitive tool. Indian J Med Paediatr Oncol 2012. [Google Scholar] [Crossref]
- PC Shah. Evaluation of peripheral lymphadenopathy by fine needle aspiration cytology: a one year study at tertiary centre. Int J Res Med Sci 2016. [Google Scholar]
- R Mohanty, A Wilkinson. Utility of Fine Needle Aspiration Cytology of Lymph nodes. IOSR J Dent Med Sci 2013. [Google Scholar]
- FI Wahid, H Rehman, Q Khan, IK Shahabi. Diagnostic value of fine needle aspiration cytology in diagnosis of non-thyroidal neck mass. JPMI 2010. [Google Scholar]
- A Shrivastav, HA Shah, PM Santwani, G Srivastava, NM Agarwal. Evaluation of peripheral lymphadenopathy by fine needle aspiration cytology: A three year study at tertiary center. J Dr. NTR Univ Health Sci 2014. [Google Scholar] [Crossref]
- JO Thomas, D Adeyi, H Amanguno. Fine-needle aspiration in the management of peripheral lymphadenopathy in a developing country. Diagn Cytopathol 1999. [Google Scholar] [Crossref]
- K Mamatha, SU Arakaru. Clinicocytological study in evaluating the primary site of tumor in patients presenting with metastatic tumors in lymph nodes. Asian J Pharma Health Sci 2014. [Google Scholar]
- A Mohan, R Thakral, S Kaur, S Singh. Fine needle aspiration in metastatic lymphadenopathy - a five year experience in Muzzafarnagar region. J Adv Res Biol Sci 2013. [Google Scholar]
- D Ghartimagar, A Ghosh, S Ranabhat, MK Shrestha, R Narasimhan, OP Talwar. Utility of fine needle aspiration cytology in metastatic lymph nodes. J Pathol Nepal 2011. [Google Scholar]
- SK Agarwal, SK Arora, G Kumar, D Sarin. Isolated perifacial lymph node metastasis in oral squamous cell carcinoma with clinically node-negative neck. Laryngoscope 2016. [Google Scholar]
- S Dowerah, R Kouli, T Karmakar. A study of FNA findings of malignancy in lymph nodes with special emphasis on metastatic malignancy. Inter J Bas Med Sci 2014. [Google Scholar]
How to Cite This Article
Vancouver
Mustaqueem SF, Hassan SB, Agarwal S. Evaluation of peripheral lymphadenopathy by fine needle aspiration cytology: Our experience at a tertiary care center [Internet]. IP Int J Med Paediatr Oncol. 2025 [cited 2025 Sep 09];7(2):67-72. Available from: https://doi.org/10.18231/j.ijmpo.2021.014
APA
Mustaqueem, S. F., Hassan, S. B., Agarwal, S. (2025). Evaluation of peripheral lymphadenopathy by fine needle aspiration cytology: Our experience at a tertiary care center. IP Int J Med Paediatr Oncol, 7(2), 67-72. https://doi.org/10.18231/j.ijmpo.2021.014
MLA
Mustaqueem, Syed Fiza, Hassan, Syed Belal, Agarwal, Shikha. "Evaluation of peripheral lymphadenopathy by fine needle aspiration cytology: Our experience at a tertiary care center." IP Int J Med Paediatr Oncol, vol. 7, no. 2, 2025, pp. 67-72. https://doi.org/10.18231/j.ijmpo.2021.014
Chicago
Mustaqueem, S. F., Hassan, S. B., Agarwal, S.. "Evaluation of peripheral lymphadenopathy by fine needle aspiration cytology: Our experience at a tertiary care center." IP Int J Med Paediatr Oncol 7, no. 2 (2025): 67-72. https://doi.org/10.18231/j.ijmpo.2021.014
