- Visibility 155 Views
- Downloads 19 Downloads
- DOI 10.18231/j.ijmpo.2020.012
-
CrossMark
- Citation
Unclassified variants of hepatic artery discovered on a 40 slice multidetector CT scanner
- Author Details:
-
Sachal Sharma *
-
Namita Singh
-
Payal Malhotra
-
Murtaza Kamal
Introduction
Liver has a dual blood supply, and receives its supply from the CHA and portal vein. CHA divides at the posterior part or pylorus or at first part of the duodenum to give rise to the gastroduodenal artery. It then advances to portal hilum as the proper hepatic artery. At hilum, the hepatic artery proper gives off the right hepatic artery entering the right lobe of liver, a left hepatic artery entering the left lobe and a small middle hepatic entering the quadrate lobe of the liver (which is not always identified).[1]
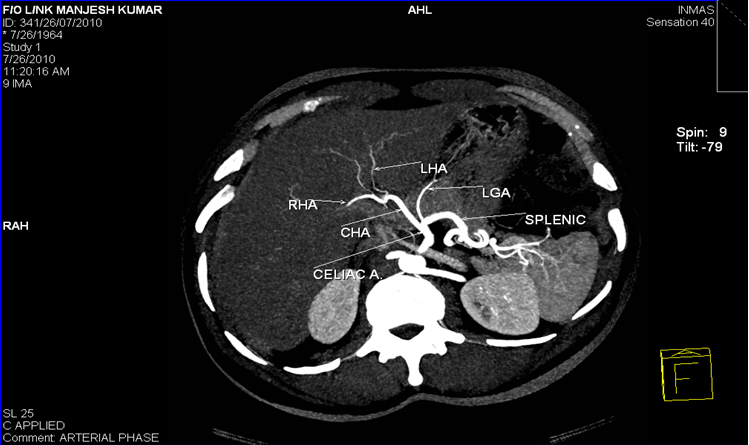
The morphologic variations in CHA may arise at its origin and /or at branching pattern with later being more common. Hepatic artery is called as an ‘aberrant hepatic artery(AHA) ‘ if it arises from a source other than the terminal end of the celiac trunk [2] and it may be accessory or replaced. The accessory hepatic artery is termed from the source of its origin. [3] The term accessory hepaticartery is called when the native left hepatic artery also exist having its origin from celiac trunk apart from the second hepatic artery supplying the same lobe. Replaced hepatic artery is termed when the hepatic artery supplying the lobe is single and not arising from celiac trunk.
Normal hepatic arterial branching system is found in 60% of the population, the rest of 40% have aberrant arterial system. Among the aberrant arterial system right hepatic artery originating from the superior mesenteric artery (10–12% of individuals) is the most common variation. The origin of common hepatic artery from the superior mesenteric artery in 2.5% of the population. The origin of left hepatic artery directly from the abdominal aorta in 2.5% of the population. [4]
Materials and Methods
A total of 110 patients underwent the study on Siemens Somatom Sensation 40. Patients underwent only post contrast acquisition of data with slice thickness of 5mm from the dome of the diaphragm till the pelvis. Thin reconstructions of images done in axial, coronal and sagittal planes. Gantry rotation of 0.5 sec per rotation with a pitch of 0.9mm used. Bolus tracking technique with an automatic injector at a rate of 3-4ml/sec. Estimated dose of contrast is 1-1.5ml/kg/body wt of the patient is used. The images obtained were transferred to a workstation for analysis. Maximum intensity projection (MIP) and multiplanar reconstruction (MPR) and volume rendered images were used for evaluation and analysis of vascular system.
Data obtained by MDCT angiography and the post processed images were evaluated by two radiologists. The average time consumption to do the post processing was app 15-20 minutes. The normal as well as the variant hepatic arterial anatomy was studied and recorded.
The initial raw data acquired from the acquisition determines the diagnostic accuracy of MDCT angiography. Accurate timing of acquisiton, proper amount of contrast injection and proper patient preparation as well as postioning play a pivotal role in study acquisition.
Results
Comparison of Michels' data with the current series ([Table 1]) shows a somewhat different incidence of variant patterns in Michels' patients. We did not observe Michels' Type X (common hepatic artery from left gastric artery), whereas Michel did not observe Hiatt Type VI (common hepatic artery from aorta). Some newer variants not described in any of the two classification system are found in the current study.
Out of total of hundred and ten (110) patients undergoing CT angiography hundred and five(105) patients had anatomy described by Michel. Five(5) patients had anatomy not described by Michels or Hiatt study.
Results are tabulated as follows
| Michels types | % in terms of Michels type | Current Study (%) |
| I | 55% | 72(68.57%) |
| II | 10% | 3(2.85%) |
| III | 11% | 7(6.66%) |
| IV | 01% | 3(2.85%) |
| V | 08% | 9(8.57%) |
| VI | 07% | 06(5.71%) |
| VII | 01% | 01(0.95%) |
| VIII | 02% | 03(2.85%) |
| IX | 2.5% | 01(0.95%) |
| X | 0.5% | 0.00 |
Results as per michels classification
| HIATT Types | HIATT Types (%) | Current Study (%) |
| I | 75.8 | 68.57 |
| II | 9.7 | 11.42 |
| III | 10.6 | 12.37 |
| IV | 2.3 | 6.65 |
| V | 1.5 | 0.95 |
| VI | 0.2 | O.95 |
NEWER VARIANTS detected in our study
1. Common hepatic artery (CHA) arising from superior mesenteric artery (SMA) and Accessory Left Hepatic Artery from Left Gastric Artery(LGA).
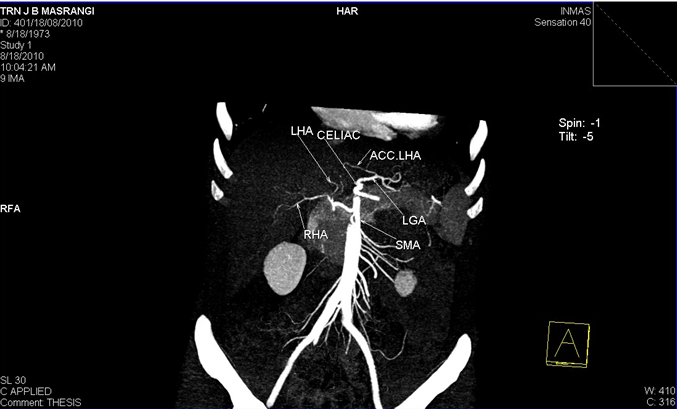
2. Right hepatic artery(RHA) and Left hepatic artery(LHA) arising directly from celiac trunk and an accessory Left Hepatic Artery (acc. LHA) from Left gastric artery(LGA).
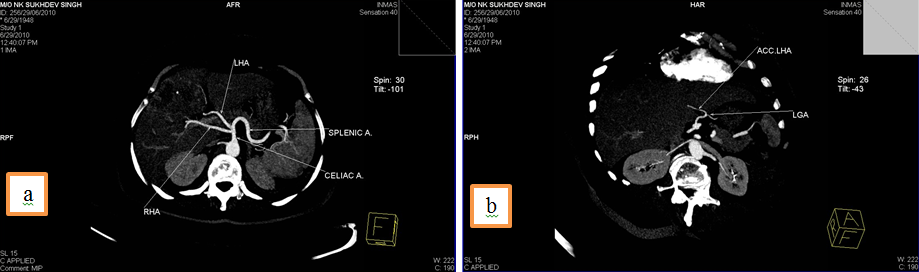
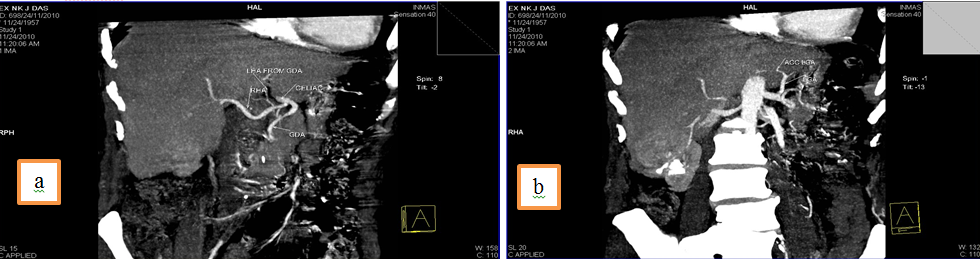
3. Common hepatic artery (CHA) arising from Superior mesenteric artery (SMA) and an accessory Right hepatic artery (acc. RHA) arising from SMA.
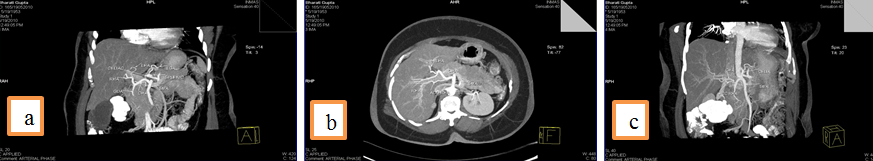
4. Left Hepatic artery (LHA) arising from Gastroduodenal artery (GDA),an accessory left hepatic artery artery arising left gastric artery and Right hepatic artery arising directly from celiac trunk.
5.Common hepatic artery (CHA) arising directly from celiac trunk, another right hepatic branch arising from celiac trunk or a double right hepatic artery and an accessory left hepatic artery arising from Left gastric artery(LGA)
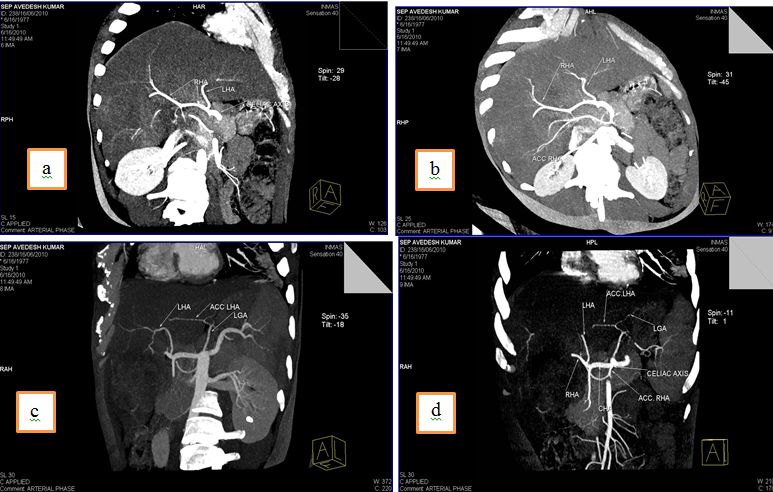
Discussion
The evolution of newer advancements and medical techniques in the management of hepatic lesions and liver transplantation has validated the recognition of normal as well as variant anatomy of the hepatic arterial system for surgical precisions and to reduce the associated co morbidities. More over the newer variants that are not described in the classification system encourage the need for a new classification that can accommodate these newer variants. The variantions in hepatic arterial system can be critical in the current advances in the treatment of hepatic pathologies.
The increasing efficacy of hepatic artery infusional chemotherapy have been shown in the recent in controlling and treating hepatic diseases. [5] During the procedure of hepatic arterial infusional chemotherapy the catheter tip is surgically placed via an arteriotomy in gastroduodenal artery at the junction of gastroduodenal and common hepatic artery. In order to achieve the goal of uniform perfusion of chemotherapeutic agent throughout the liver the recognition of any variant anatomy of hepatic arterial system is essential so that the procedure can be manipulated. [5] In the patients with variant arterial anatomy, vascular reconstruction or use of double port catheter pumps are used to attain uniform perfusion. [6]
Double hepatic arterial variant which is not included in the Michels classification has a particular relevance with respect to catheter position and number of catheters so as to achieve an ideal uniform distribution of chemotherapeutic agent.[7]
In the clinical scenario of a liver transplant patient apart from hepatic and portal venous anatomy, hepatic arterial anatomic variations play a pivotal role. All hepatic arterial variations need to be evaluated for preservation and revasculaization to avoid parenchymal ischemic and biliary tract abnormalities. [8] Adequate knowledge of hepatic arterial anatomy is very crucial for selection of patients for living related liver transplantation(LRLT) and MDCT is the mainstay in preoperative evaluation of classical and variable hepatic arterial anatomy. Knowing the left lobe arterial supply and segment IV arterial supply is critical in ensuring optimal donor hepatectomy and proper graft vascularization. The major concern in hepatic arterial variation in preoperative evaluation is aberrant LHA arising from left gastric artery, in the cases where left lobe of liver is used as a graft. [9] Moreover surgical approach can be modified as per the the preoperative knowledge of anomalous vessels. [10] During the transplant operative procedures post operative arterial complications can be anticipated with more complicated graft reconstructions and appropriate post op measures can be taken to overcome them.[8] Multiple arterial anastomosis can lead to technical difficulties that can lead to multiple complications such as biliary stricture, cholangitis, cholangiolar abscesses and graft rejection as the biliary ducts are vascularized only by the hepatic arteries. [11] Segment IV artery arising from right hepatic artery is important in case of right lobe liver transplantation as it need to be spared during the operative procedure so as not to induce ischemic changes in the donor liver. [12]
Trauma to hepatic parenchyma and hepatic arterial trauma is a known entity durng blunt trauma abdomen. [13] In case of traumatic liver hematoma arising from an aberrant hepatic artery it may pose a potential diagnostic error during conventional angiography.[14] Preoperative evaluation of hepatic arterial anatomy has attained a pivotal role in laparoscopic cholecystectomy. [11] Biliary and vascular injuries though uncommon, but are known entities encountered in laproscopic cholecystectomy. [15] Variant arterial anatomy increases the chances of such arterial injuries. Knowing the hepatic arterial anatomy can very well avoid the arterial injury and measures can be taken to preserve the vessels. [16]
Source of Funding
None.
Conflict of Interest
None.
References
- H Gray, Mwj Ferguson, L H Bannister, M M Berry, M Dyson, P Collins, J E Dussek. Abdominal aorta. 1995. [Google Scholar]
- N A Michels. Blood supply and anatomy of the upper abdominal organs with a descriptive atlas. Philadelphia: JB Lippincott. 1955. [Google Scholar]
- J E Healey, P C Schroy, R J Serensen. The intrahepatic distribution of the hepatic artery in man. J Internat Coll Surg 1953. [Google Scholar]
- H Tuncay. Mesenteric Arterial Variations Detected at MDCT Angiography of Abdominal AortaDOI. AJR 2009. [Google Scholar]
- Corinne B. Winston, Nancy A. Lee, William R. Jarnagin, Jerrold Teitcher, Ronald P. DeMatteo, Yuman Fong. CT Angiography for Delineation of Celiac and Superior Mesenteric Artery Variants in Patients Undergoing Hepatobiliary and Pancreatic Surgery. American Journal of Roentgenology 2007. [Google Scholar]
- A Eid, P Reissman, G Zamir, A J Pikarsky. Reconstruction of replaced right hepatic artery, to implant a single-catheter port for intra-arterial hepatic chemotherapy. Am Surg 1998. [Google Scholar]
- A M Convey, Lynn A. Brody, Mary A. Maluccio, George I. Getrajdman, Karen T. Brown. Variant Hepatic Arterial Anatomy Revisited: Digital Subtraction Angiography Performed in 600 patients. Int Radiol J 2002. [Google Scholar]
- A. S. Soin, P. J. Friend, A. Rasmussen, R. Saxena, Y. Tokat, G. J. M. Alexander. Donor arterial variations in liver transplantation: Management and outcome of 527 consecutive grafts. Br J Surg 1996. [Google Scholar]
- S Todo, L Makowka, A G Tzakis. Hepatic artery in liver transplantation. Transplant Proc 1987. [Google Scholar]
- C M Volpe, S Peterson, E L Hoover, R J Doerr. Justification for visceral angiography prior to pancreaticoduodenectomy. Am Surg 1988. [Google Scholar]
- R M Jones. The hepatic artery:a reminder of surgical anatomy. J R Coll Surg Edinb 2001. [Google Scholar]
- Cihan Duran, Suleyman Uraz, Mecit Kantarci, Ersin Ozturk, Selim Doganay, Murat Dayangac. Hepatic Arterial Mapping by Multidetector Computed Tomographic Angiography in Living Donor Liver Transplantation. Journal of Computer Assisted Tomography 2009. [Google Scholar]
- . Mesenteric artery, secondary to blunt trauma. J Trauma 2000. [Google Scholar]
- Marvin A. Konstam, Robert A. Novelline, Christos A. Athanasoulis. Aberrant hepatic artery: A potential cause for error in the angiographic diagnosis of traumatic liver hematoma. Gastrointest Radiol 1979. [Google Scholar]
- Daniel J. Deziel, Keith W. Millikan, Steven G. Economou, Alexander Doolas, Sung-Tao Ko, Mohan C. Airan. Complications of laparoscopic cholecystectomy: A national survey of 4,292 hospitals and an analysis of 77,604 cases. Am J Surg 1993. [Google Scholar]
- Thomas R. Biehl, L. William Traverso, Ellen Hauptmann, John A. Ryan. Preoperative visceral angiography alters intraoperative strategy during the whipple procedure. Am J Surg 1993. [Google Scholar]
How to Cite This Article
Vancouver
Sharma S, Singh N, Malhotra P, Kamal M. Unclassified variants of hepatic artery discovered on a 40 slice multidetector CT scanner [Internet]. IP Int J Med Paediatr Oncol. 2025 [cited 2025 Sep 05];6(2):52-55. Available from: https://doi.org/10.18231/j.ijmpo.2020.012
APA
Sharma, S., Singh, N., Malhotra, P., Kamal, M. (2025). Unclassified variants of hepatic artery discovered on a 40 slice multidetector CT scanner. IP Int J Med Paediatr Oncol, 6(2), 52-55. https://doi.org/10.18231/j.ijmpo.2020.012
MLA
Sharma, Sachal, Singh, Namita, Malhotra, Payal, Kamal, Murtaza. "Unclassified variants of hepatic artery discovered on a 40 slice multidetector CT scanner." IP Int J Med Paediatr Oncol, vol. 6, no. 2, 2025, pp. 52-55. https://doi.org/10.18231/j.ijmpo.2020.012
Chicago
Sharma, S., Singh, N., Malhotra, P., Kamal, M.. "Unclassified variants of hepatic artery discovered on a 40 slice multidetector CT scanner." IP Int J Med Paediatr Oncol 6, no. 2 (2025): 52-55. https://doi.org/10.18231/j.ijmpo.2020.012
